Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 He spin filter and small-angle X-ray scattering
He spin filter and small-angle X-ray scatteringRyoki, Akiyuki*; Watanabe, Fumi*; Okudaira, Takuya*; Takahashi, Shingo*; Oku, Takayuki; Hiroi, Kosuke; Motokawa, Ryuhei; Nakamura, Yo*
Journal of Chemical Physics, 160(11), p.114907_1 - 114907_9, 2024/03
Sato, Nobuaki*; Kirishima, Akira*; Sasaki, Takayuki*; Takano, Masahide; Kumagai, Yuta; Sato, Soichi; Tanaka, Kosuke
Current Location of Fuel Debris Chemistry, 178 Pages, 2023/11
Considerable efforts have been devoted to the decommissioning of the TEPCO's Fukushima Daiichi Nuclear Power Station (1F) and now the retrieval of fuel debris is being proceeded on a trial basis. It can be said that the succession of science and technology related to debris, that is, human resource development, is important and indispensable. For that reason, we thought that a specific textbook on decommissioning is necessary. Regarding the 1F fuel debris, we still do not know enough, and it would be difficult to describe the details. However, 12 years have passed since the accident, and we have come to understand the situation of 1F to a certain extent. At this stage, it is essential for future development to organize the current situation by combining examples of past severe accidents. Therefore, we presented in this book the current state of fuel debris chemistry research from the perspectives of solid chemistry, solution chemistry, analytical chemistry, radiochemistry, and radiation chemistry.
Soma, Yasutaka; Komatsu, Atsushi; Ueno, Fumiyoshi
Corrosion, 78(6), p.503 - 515, 2022/06
Times Cited Count:0 Percentile:0(Materials Science, Multidisciplinary)The effects of electrochemical potential (ECP) on water chemistry within a crevice are of critical importance for understanding stress corrosion cracking (SCC) of Fe-Cr-Ni alloys in high temperature water. In this study, the effects of ECP on the electrical conductivity of a solution within a Type-316L stainless steel crevice (
 ) have been studied in 288
) have been studied in 288 C and 8 MPa water containing 10 ppb Cl
C and 8 MPa water containing 10 ppb Cl as major anionic species. In situ measurements of
as major anionic species. In situ measurements of 
 in a rectangular crevice with a gap of 15
in a rectangular crevice with a gap of 15  m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in
m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in 
 from 12
from 12  Scm
Scm to 160
to 160  Scm
Scm at a distance of 21 mm from the crevice mouth. The increase in
at a distance of 21 mm from the crevice mouth. The increase in 
 reached a maximum at about 0.15 V (about 300
reached a maximum at about 0.15 V (about 300  Scm
Scm ) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl
) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl is the major anionic species transported into the crevice at relatively low potentials, and that
is the major anionic species transported into the crevice at relatively low potentials, and that 
 increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
 generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
 mesocrystal formation
mesocrystal formationLi, Z.*; Piankova, D.*; Yang, Y.*; Kumagai, Yuta; Zschiesche, H.*; Jonsson, M.*; Tarakina, N. V.*; Soroka, I. L.*
Angewandte Chemie; International Edition, 61(6), p.e202112204_1 - e202112204_9, 2022/02
Times Cited Count:4 Percentile:30.82(Chemistry, Multidisciplinary)The role of intermediate phases in CeO mesocrystal formation from aqueous Ce(III) solutions subjected to
mesocrystal formation from aqueous Ce(III) solutions subjected to  -radiation was studied. Radiolytically formed hydroxyl radicals convert soluble Ce(III) into less soluble Ce(IV). Transmission electron microscopy and X-ray diffraction studies of samples from different stages of the process allowed the identification of several stages in CeO
-radiation was studied. Radiolytically formed hydroxyl radicals convert soluble Ce(III) into less soluble Ce(IV). Transmission electron microscopy and X-ray diffraction studies of samples from different stages of the process allowed the identification of several stages in CeO mesocrystal evolution following the oxidation to Ce(IV): (1) formation of hydrated Ce(IV)-hydroxides, serving as intermediates in the liquid-to-solid phase transformation; (2) CeO
mesocrystal evolution following the oxidation to Ce(IV): (1) formation of hydrated Ce(IV)-hydroxides, serving as intermediates in the liquid-to-solid phase transformation; (2) CeO primary particle growth inside the intermediate phase; (3) alignment of the primary particles into "pre-mesocrystals" and subsequently to mesocrystals, guided by confinement of the amorphous intermediate phase and accompanied by the formation of mineral bridges. Further alignment of the obtained mesocrystals into supracrystals occurs upon slow drying, making it possible to form complex hierarchical architectures.
primary particle growth inside the intermediate phase; (3) alignment of the primary particles into "pre-mesocrystals" and subsequently to mesocrystals, guided by confinement of the amorphous intermediate phase and accompanied by the formation of mineral bridges. Further alignment of the obtained mesocrystals into supracrystals occurs upon slow drying, making it possible to form complex hierarchical architectures.
Zhang, W. Q.*; Yamaguchi, Toshio*; Fang, C. H.*; Yoshida, Koji*; Zhou, Y. Q.*; Zhu, F. Y.*; Machida, Shinichi*; Hattori, Takanori; Li, W.*
Journal of Molecular Liquids, 348, p.118080_1 - 118080_11, 2022/02
Times Cited Count:2 Percentile:34.79(Chemistry, Physical)The ion hydration and association and hydrogen-bonded water structure in an aqueous 3 mol/kg RbCl solution were investigated at 298 K/0.1 MPa, 298 K/1 GPa, 523 K/1 GPa, and 523 K/4 GPa by neutron diffraction combined with EPSR methods. The second hydration layer of Rb and Cl
and Cl becomes evident under elevated pressure and temperature conditions. The average oxygen coordination number of Rb
becomes evident under elevated pressure and temperature conditions. The average oxygen coordination number of Rb (Cl
(Cl ) in the first hydration layer increases from 6.3 (5.9) ambient pressure to 8.9 (9.1) at 4 GPa, while decreasing coordination distance from 0.290 nm (0.322 nm) to 0.288 nm (0.314 nm). The orientation of the water dipole in the first solvation shell of Rb
) in the first hydration layer increases from 6.3 (5.9) ambient pressure to 8.9 (9.1) at 4 GPa, while decreasing coordination distance from 0.290 nm (0.322 nm) to 0.288 nm (0.314 nm). The orientation of the water dipole in the first solvation shell of Rb and a central water molecule is sensitive to pressure, but that in the first solvation shell of Cl
and a central water molecule is sensitive to pressure, but that in the first solvation shell of Cl does not change very much. The number of contact-ion pairs Rb
does not change very much. The number of contact-ion pairs Rb -Cl
-Cl decreases with elevated temperature and increases with elevated pressure. Water molecules are closely packed, and the tetrahedral hydrogen-bonded network of water molecules no longer exists in extreme conditions.
decreases with elevated temperature and increases with elevated pressure. Water molecules are closely packed, and the tetrahedral hydrogen-bonded network of water molecules no longer exists in extreme conditions.
Tominaga, Taiki*; Sahara, Masae*; Kawakita, Yukinobu; Nakagawa, Hiroshi; Yamada, Takeshi*
Journal of Applied Crystallography, 54(6), p.1631 - 1640, 2021/12
Times Cited Count:4 Percentile:55.82(Chemistry, Multidisciplinary)Igarashi, Takahiro; Otani, Kyohei; Kato, Chiaki; Sakairi, Masatoshi*; Togashi, Yusuke*; Baba, Kazuhiko*; Takagi, Shusaku*
ISIJ International, 61(4), p.1085 - 1090, 2021/04
Times Cited Count:1 Percentile:8.06(Metallurgy & Metallurgical Engineering)In order to clarify the effect of metal cations (Zn , Mg
, Mg , Na
, Na ) in aqueous solution on hydrogen permeation into iron, the amount of hydrogen permeation from iron surface was measured by electrochemical tests with a laser ablation. Moreover, in order to obtain the basic mechanism of hydrogen permeation with metal cation, first-principles calculations were used to acquire the adsorption potential of the metal cation and the electronic state around iron surface. By Zn
) in aqueous solution on hydrogen permeation into iron, the amount of hydrogen permeation from iron surface was measured by electrochemical tests with a laser ablation. Moreover, in order to obtain the basic mechanism of hydrogen permeation with metal cation, first-principles calculations were used to acquire the adsorption potential of the metal cation and the electronic state around iron surface. By Zn in solution, anodic reaction on ablated surface by laser irradiation was suppressed. Also, by quantum analysis Zn atoms were chemically bonded stronger than Na and Mg atoms to iron surface. It was suggested that the dissolution reaction of iron was suppressed by the formation of the Zn layer, and that lead suppression of hydrogen permeation into iron.
in solution, anodic reaction on ablated surface by laser irradiation was suppressed. Also, by quantum analysis Zn atoms were chemically bonded stronger than Na and Mg atoms to iron surface. It was suggested that the dissolution reaction of iron was suppressed by the formation of the Zn layer, and that lead suppression of hydrogen permeation into iron.
 O
O -type Schiff base complexes of tetravalent f-elements (M(IV) = Ce, Th, U, Np, and Pu) in solid state and in solution
-type Schiff base complexes of tetravalent f-elements (M(IV) = Ce, Th, U, Np, and Pu) in solid state and in solutionRadoske, T.*; Kloditz, R.*; Fichter, S.*; M rz, J.*; Kaden, P.*; Patzschke, M.*; Schmidt, M.*; Stumpf, T.*; Walter, O.*; Ikeda, Atsushi
rz, J.*; Kaden, P.*; Patzschke, M.*; Schmidt, M.*; Stumpf, T.*; Walter, O.*; Ikeda, Atsushi
Dalton Transactions (Internet), 49(48), p.17559 - 17570, 2020/12
Times Cited Count:10 Percentile:65.05(Chemistry, Inorganic & Nuclear)Segawa, Tomoomi; Kawaguchi, Koichi; Ishii, Katsunori; Suzuki, Masahiro; Fukasawa, Tomonori*; Fukui, Kunihiro*
Funtai Kogakkai-Shi, 57(9), p.485 - 494, 2020/09
In the spent fuel reprocessing process, a mixed solution of uranyl nitrate and plutonium nitrate is converted into mixed oxide powder by the microwave heating. To evaluate the applicability to the industrial-scale and acquire the characteristics data of the microwave heating denitration of various metal nitrate aqueous solutions based on the knowledge studied in the development of laboratory-scale basic experiments, the microwave heating characteristics and metal oxide powder properties were investigated using cerium nitrate, cobalt nitrate and copper nitrate aqueous solutions. The progress rate of the denitration reaction was depended on the position, and the denitration reaction proceeded faster at the periphery than at the center. The morphologies of the synthesized products were porous and hard dry solid with cerium nitrate aqueous solution, foamed dry solid with cobalt nitrate aqueous solution, and powdery particles with copper nitrate aqueous solution. The denitration ratio and average particle size of the synthesized products increased in the order of the cerium nitrate aqueous solution, the cobalt nitrate aqueous solution, and the copper nitrate aqueous solution. The numerical simulations revealed that the periphery of the bottom surface of the metal nitrate aqueous solution was heated by microwaves. This results consistent with the experimental results in which the denitration reaction started from the periphery of the metal nitrate aqueous solution.
 -radiation and H
-radiation and H O
O induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acid
induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acidKumagai, Yuta; Jonsson, M.*
Dalton Transactions (Internet), 49(6), p.1907 - 1914, 2020/02
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)This study aims to reveal possible involvements of organic acids in the oxidative dissolution of UO . Using phthalic acid as a model compound, we have measured adsorption on UO
. Using phthalic acid as a model compound, we have measured adsorption on UO and investigated effects on the reaction between H
and investigated effects on the reaction between H O
O and UO
and UO and on oxidative dissolution induced by
and on oxidative dissolution induced by  -irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H
-irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H O
O and UO
and UO in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO
in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO surface, it is not involved in the interfacial reaction by H
surface, it is not involved in the interfacial reaction by H O
O . In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H
. In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H O
O generated by radiolysis was consumed by UO
generated by radiolysis was consumed by UO . The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO
. The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO .
.
Tsuji, Masakuni*; Nakashima, Hitoshi*; Saito, Akira*; Okihara, Mitsunobu*; Sato, Toshinori
45th Annual Waste Management Conference (WM 2019); Encouraging Young Men & Women to Achieve Their Goals in Radwaste Management, Vol.7, p.4749 - 4763, 2020/01
A rock excavation grouting technology has been recently studied as significant technology for reducing the ingress of water into the deep repository. However, it has not been studied for applying to the coastal region, where it is discussed to be a more suitable region for the geological disposal in Japan. The latest material called colloidal silica grout (CSG) is good for sealing narrow fractures but is known to be sensitive to the salinity of groundwater because of its gelling property with salt accelerator. Although the gelling of CSG can be controlled by adding an acidic pH adjuster, the methodology for delivering the appropriate grout is not well established for such conditions of saline groundwater. Therefore, this research project was established to enhance the existing rock grouting technology for deep repositories.
Sasaki, Yuji; Ban, Yasutoshi; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
Solvent Extraction Research and Development, Japan, 27(1), p.63 - 67, 2020/00
Times Cited Count:6 Percentile:31.74(Chemistry, Multidisciplinary)Mutual separation technique of Dy and Nd in Nd magnet is studied. Dy is more valuable than Nd, then Dy might be isolated and reused. Lanthanide elements can be extracted thoroughly by diglycolamide (DGA) extractants, we use this reagent for the recovery and isolation of Dy. Tetradodecyl-DGA (TDdDGA) has relatively high separation factors(SF) between Dy and Nd (SF=17-18) in HNO extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO
extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO , 92% Dy can be recovered with 0.7% co-extraction of Nd.
, 92% Dy can be recovered with 0.7% co-extraction of Nd.
Yamaguchi, Toshio*; Nishino, Masaaki*; Yoshida, Koji*; Takumi, Masaharu*; Nagata, Kiyofumi*; Hattori, Takanori
European Journal of Inorganic Chemistry, 2019(8), p.1170 - 1177, 2019/02
Times Cited Count:16 Percentile:74.57(Chemistry, Inorganic & Nuclear)Neutron diffraction measurements of an aqueous 2 mol dm CaCl
CaCl solutions in D
solutions in D O have been made at 1 GPa, 298 K as well as 0.1 MPa, 298 K. The experimental structure factors are subjected to Empirical Potential Structure Refinement (EPSR) modeling to reveal the ion hydration and association and solvent water at the atomic level. About seven water molecules surround Ca
O have been made at 1 GPa, 298 K as well as 0.1 MPa, 298 K. The experimental structure factors are subjected to Empirical Potential Structure Refinement (EPSR) modeling to reveal the ion hydration and association and solvent water at the atomic level. About seven water molecules surround Ca at the Ca-O and Ca-D distances of 2.44
at the Ca-O and Ca-D distances of 2.44  and 3.70
and 3.70  , respectively, at both pressures, suggesting no significant pressure effect on the cation hydration. On the other hand, the Cl
, respectively, at both pressures, suggesting no significant pressure effect on the cation hydration. On the other hand, the Cl ion shows a drastic change in water oxygen coordination from 7 at 0.1 MPa to 14 at 1 GPa, accompanied by shortening of Cl-O distance from 3.18
ion shows a drastic change in water oxygen coordination from 7 at 0.1 MPa to 14 at 1 GPa, accompanied by shortening of Cl-O distance from 3.18  to 3.15
to 3.15  . However, the number of water hydrogen atoms around Cl
. However, the number of water hydrogen atoms around Cl does not change significantly as 6.0
does not change significantly as 6.0  6.7 with shortening Cl-D distance from 2.22 to 2.18
6.7 with shortening Cl-D distance from 2.22 to 2.18  on compression. The pressure effect on the solvent water structure is also drastic as an increase in water oxygen atoms of 4.7 at the O-O distance of 2.79
on compression. The pressure effect on the solvent water structure is also drastic as an increase in water oxygen atoms of 4.7 at the O-O distance of 2.79  at 0.1 MPa to 10.3 at 2.85
at 0.1 MPa to 10.3 at 2.85  at 1 GPa. The number of water hydrogen atoms, however, does not change as 1.2 at the O-D distance of 1.74
at 1 GPa. The number of water hydrogen atoms, however, does not change as 1.2 at the O-D distance of 1.74  for both pressures, demonstrating the presence of the O
for both pressures, demonstrating the presence of the O D hydrogen bonds which are significantly bent at 1 GPa at 298 K. This change of hydrogen bonds in water with pressure probably causes the drastic increase in water oxygen atoms around Cl
D hydrogen bonds which are significantly bent at 1 GPa at 298 K. This change of hydrogen bonds in water with pressure probably causes the drastic increase in water oxygen atoms around Cl .
.
Fujita, Shunya*; Abe, Yutaka*; Kaneko, Akiko*; Yuasa, Tomohisa*; Segawa, Tomoomi; Kato, Yoshiyuki; Kawaguchi, Koichi; Ishii, Katsunori
Proceedings of 11th Korea-Japan Symposium on Nuclear Thermal Hydraulics and Safety (NTHAS-11) (Internet), 7 Pages, 2018/11
Mixed uranium oxide and plutonium oxide powder is produced from uranyl nitrate and plutonium nitrate mixed solution by the microwave heating denitration method in the spent fuel reprocessing process. Since the microwave heating method is accompanied by a boiling phenomenon, it is necessary to fully grasp the operating conditions in order to avoid flashing and spilling in the mass production of denitrification technology for the future. In this research, it was clarified that the heat transfer coefficient became lower as the dielectric constant increased. The dominant factor of the blowing up phenomena is supposed to be generation of the innumerable bubble rather than bubble's growth.
Toguri, Satohito*; Okihara, Mitsunobu*; Tsuji, Masakuni*; Nakashima, Hitoshi*; Sugiyama, Hirokazu*; Saito, Akira*; Sato, Toshinori; Aoyagi, Kazuhei; Masunaga, Kosuke
JAEA-Research 2017-013, 131 Pages, 2018/02
The discussions on scientifically promising site for the geological disposal has been made at the council of studying group on techniques for geological disposal of radioactive wastes, which is held by Resources and Energy Agency. From the aspect of ensuring safety during the transportation of disposal waste, the coastal area is discussed to be a more suitable area. This report shows the result of the first year of this project as following items; Study on the state-of-art technology and remain tasks; laboratory tests on characterization of colloidal silica grout under sea water; Study on the development of grouting technology (design and the evaluation method of influence on the rock mass).
 , and NaCO
, and NaCO solutions
solutionsUehara, Akihiro*; Fujii, Toshiyuki*; Yamana, Hajimu*; Okamoto, Yoshihiro
Radiochimica Acta, 104(1), p.1 - 9, 2016/01
Times Cited Count:7 Percentile:55.03(Chemistry, Inorganic & Nuclear)The spectroelectrochemical cell was fabricated for in-situ X-ray absorption fine structure (XAFS) measurement. The XAFS spectra of U L -edge were monitored in electrolyte solutions during the electrochemical reduction. Tetravalent uranium was electrochemically prepared from hexavalent uranium and the extended X-ray absorption fine structure (EXAFS) was analyzed. The U
-edge were monitored in electrolyte solutions during the electrochemical reduction. Tetravalent uranium was electrochemically prepared from hexavalent uranium and the extended X-ray absorption fine structure (EXAFS) was analyzed. The U formed in 0.1M HNO
formed in 0.1M HNO was partially re-oxidized to UO
was partially re-oxidized to UO
 by NO
by NO
 in the solution. The UO
in the solution. The UO
 carbonates were prepared during electrolysis.
carbonates were prepared during electrolysis.
 -ray irradiation
-ray irradiationMotooka, Takafumi; Ueno, Fumiyoshi
Zairyo To Kankyo, 64(6), p.220 - 223, 2015/06
Corrosion behavior of carbon steel in chloride aqueous solutions under a low dose rate was investigated by corrosion test using chloride aqueous solutions with different chloride concentration at a dose rate of 500 Gy/h. The corrosion rate of carbon steel increased by the irradiation, and the corrosion rate had the maximum value at a certain chloride concentration. The oxidants produced by radiolysis of chloride aqueous solution enhanced the corrosion of carbon steel. The main oxidants were oxygen and hydrogen peroxide, and the diffusion process of oxidants controlled the corrosion of carbon steel under irradiation. There was a positive correlation between the dependence of corrosion rate and chloride concentration and the dependence of oxidant concentration and chloride concentration.
Oe, Kazuhiro*; Attallah, M. F.*; Asai, Masato; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke*; Kasamatsu, Yoshitaka*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1317 - 1320, 2015/02
Times Cited Count:10 Percentile:64.63(Chemistry, Analytical)A new technique for continuous dissolution of nuclear reaction products transported by a gas-jet system was developed for superheavy element (SHE) chemistry. In this technique, a hydrophobic membrane is utilized to separate an aqueous phase from the gas phase. With this technique, the dissolution efficiencies of short-lived radionuclides of 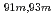 Mo and
Mo and  W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
Arisaka, Makoto; Kimura, Takaumi; Nagaishi, Ryuji; Yoshida, Zenko
Journal of Alloys and Compounds, 408-412, p.1307 - 1311, 2006/02
Times Cited Count:6 Percentile:42.74(Chemistry, Physical)no abstracts in English
Kimura, Takaumi; Kirishima, Akira*; Arisaka, Makoto
Kidorui, (47), p.43 - 56, 2005/11
no abstracts in English